REVIEW ARTICLE | https://doi.org/10.5005/jp-journals-10082-02215 |
Histopathological Spectrum of Granulomatous Skin Lesions: A Review
1,2Department of Pathology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth Deemed University, Puducherry, India
Corresponding Author: Sowmya Srinivasan, Department of Pathology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth Deemed University, Puducherry, India, Phone: +91 8903602824, e-mail: drssowmya@hotmail.com
How to cite this article George VP, Srinivasan S. Histopathological Spectrum of Granulomatous Skin Lesions: A Review. J Basic Clin Appl Health Sci 2019;2(3):95–104.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background and objectives: Granulomatous dermatoses are the most frequently encountered dermatological disorders in tropical countries with leprosy and tuberculosis as the leading causes. Although granulomatous skin lesions are very prevalent in society today, dermatopathologists often face diagnostic challenges while reporting these lesions due to the similar histopathological features or tissue reaction patterns seen in these lesions with diverse etiologies. The purpose of this study is to review the various causes and patterns of granulomatous skin lesions and a sequential approach to diagnosis of individual lesions accurately.
Materials and methods: A comprehensive search of all literature available on granulomatous skin diseases between 2000 and 2018 was conducted, using keywords such as “granulomatous dermatosis,” “cutaneous tuberculosis,” “leprosy,” and “histopathological study” in the databases such as PubMed, ProQuest, and Google Scholar. The articles that satisfied the criteria were included in the final review.
Results: The available evidence from the existing systematic literature reviews and articles suggested that the various tissue reaction patterns observed in granulomatous skin lesions are classified as epithelioid, palisaded, suppurative, xanthogranulomatous, foreign body, and other granulomatous patterns. These tissue reaction patterns are caused by infections, foreign bodies, metabolites, chemicals, and malignancies.
Conclusion: Granulomatous dermatoses are a heterogeneous group of skin disorders that are frequently encountered in diagnostic dermatopathology. The histomorphology of a granuloma has to be correlated with the clinical history and ancillary techniques including special stains, culture, and molecular methods are necessary to arrive at a definitive diagnosis.
Keywords: Cutaneous tuberculosis, Granulomatous dermatosis, Histopathological study, Leprosy.
INTRODUCTION
Granulomatous skin lesions often present as a diagnostic challenge to dermatopathologists as a single identical histopathological pattern can be produced by several etiologies and contrariwise, a single etiology may produce diverse histolopathological patterns.1 A granulomatous inflammation is a chronic inflammatory response with a distinctive tissue reaction pattern defined by focal clusters of epithelioid histiocytes, multinucleated giant cells, and mononuclear leukocytes. This is a type IV or delayed hypersensitivity reaction induced by infection, reactions to autoimmunity, toxins, allergies, drugs, and neoplasms.2 The cardinal tissue reaction patterns seen in granulomatous skin lesions are predominantly epithelioid granulomas, and there are varied morphological patterns which will be discussed in the subsequent sections.3
MATERIALS AND METHODS
In this article, we have conducted a comprehensive literature search in PubMed, ProQuest, and Google Scholar for articles published on granulomatous skin lesions during 2000–2018. Literature search was conducted using key words such as “granulomatous dermatosis,” “cutaneous tuberculosis,” “leprosy,” and “histopathological study” in combination or in isolation.
Few algorithms for approach to diagnosis of granulomas are illustrated in Flowcharts 1 to 4.4
Granulomas are discussed under the subtypes of epithelioid, palisaded, suppurative, xanthogranuloma, foreign body type, and miscellaneous entities mimicking granulomatous conditions.5
EPITHELIOID GRANULOMAS
Epithelioid granulomas consist of epithelioid histiocytes or macrophages, few of which fuse to form large multinucleated giant cells admixed with lymphocytes and occasional plasma cells with or without features of necrosis. A variety of conditions showing epithelioid granulomas are discussed in the ensuing sections.6

Flowchart 1: Algorithmic approach to diagnosis of granulomas
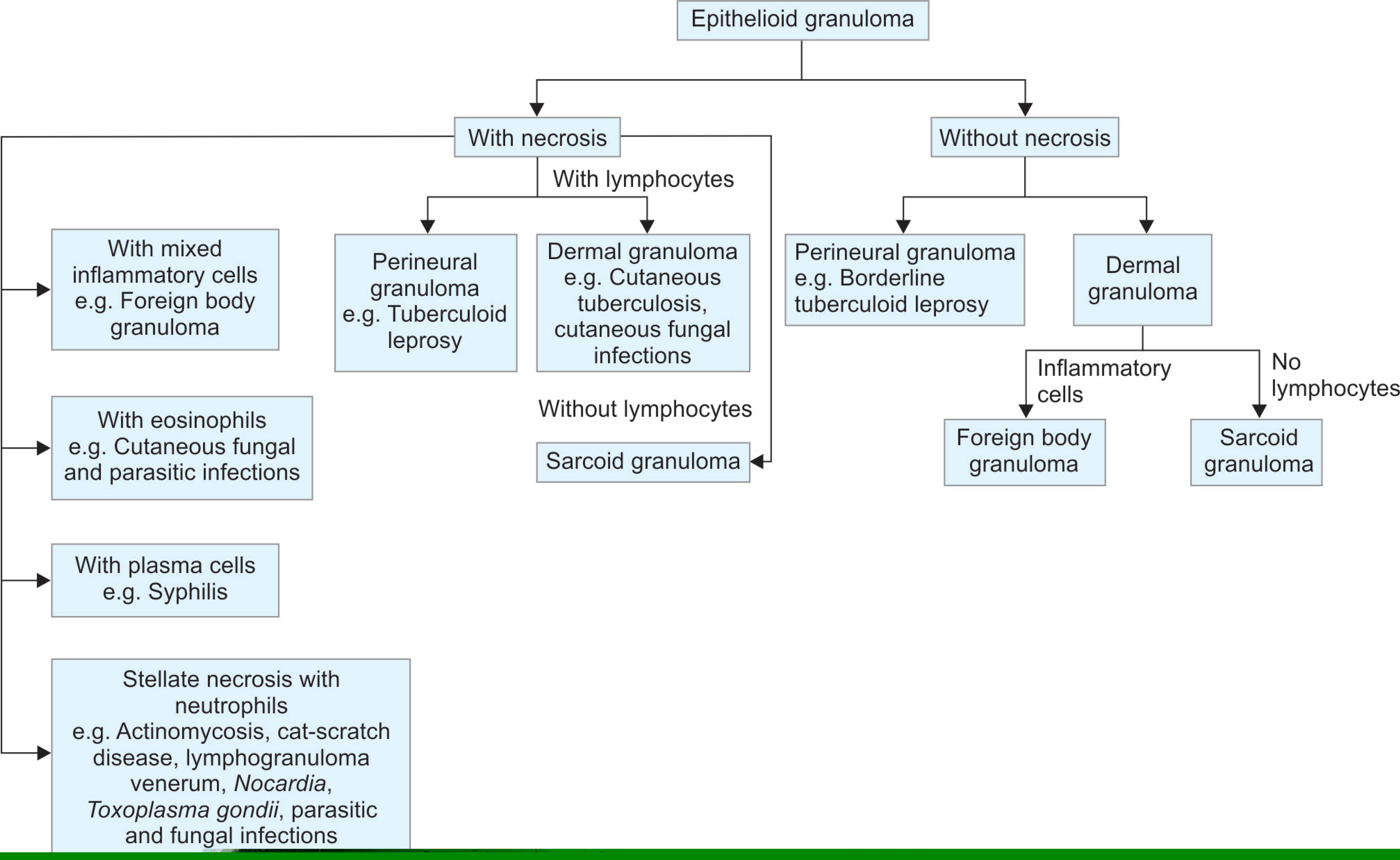
Flowchart 2: Algorithmic approach to diagnosis of epithelioid granulomas with and without necrosis
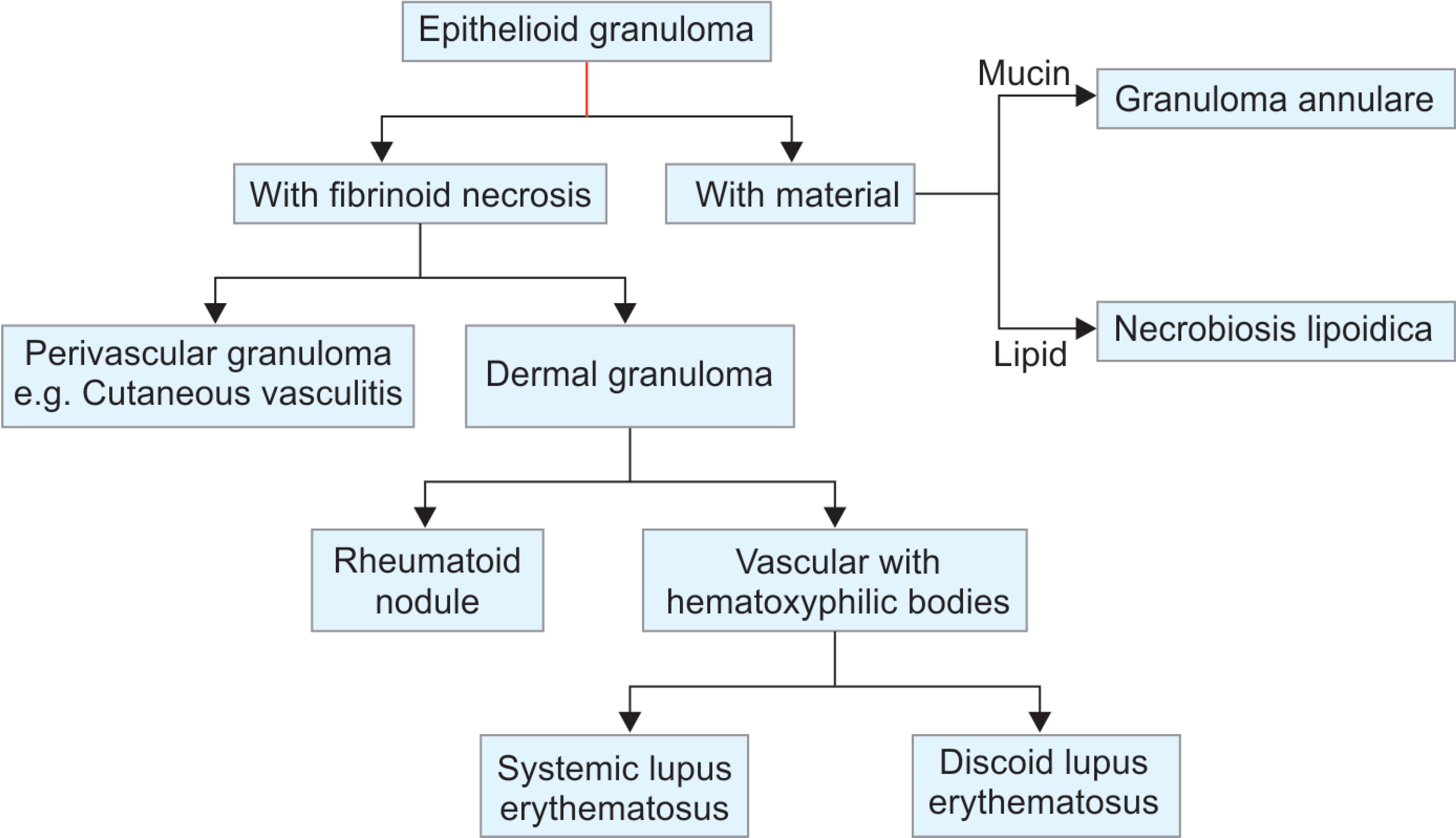
Flowchart 3: Algorithmic approach to diagnosis of epithelioid granulomas with fibrinoid necrosis and epithelioid granulomas with endogenous material
Leprosy is a chronic infectious granulomatous condition caused by Mycobacterium leprae that is endemic in many regions of the world. Leprosy can affect skin and peripheral nerves and usually patient presents clinically with macular, nodular, infiltrative, or diffuse lesions.7 Depending on the host’s immunity, it has varied histopathological appearances. Epithelioid granulomas are seen with lymphocytic infiltration of the sweat glands, neurovascular bundles, and erector pili muscle in the tuberculoid variant. The borderline tuberculoid variant has discrete granulomas (Fig. 1) with clear Grenz zone.8 Polar tuberculoid leprosy shows confluent granulomas with basal layer erosion, and there may be necrosis and giant cells resembling tuberculous granulomas (Fig. 2).9 Fite–Faraco stain is applied for diagnostic purpose to demonstrate the acid-fast lepra bacilli, which are usually scanty.10
Lupus vulgaris is a chronic progressive form of cutaneous tuberculosis. The lesions are small red-brown-colored gelatinous papules (apple-jelly nodules) that gradually evolve into large plaques as a result of central atrophy and extension to the periphery. Histologically, there is granulomatous inflammation composed of giant cells in the mid-dermis with or without caseating necrosis (Fig. 3). Acid-fast bacilli (AFB) are usually found in the areas of necrosis by use of special stains [Ziehl–Neelsen (ZN)]. Culture and molecular techniques such as polymerase chain reaction (PCR) may be required for diagnosis.11
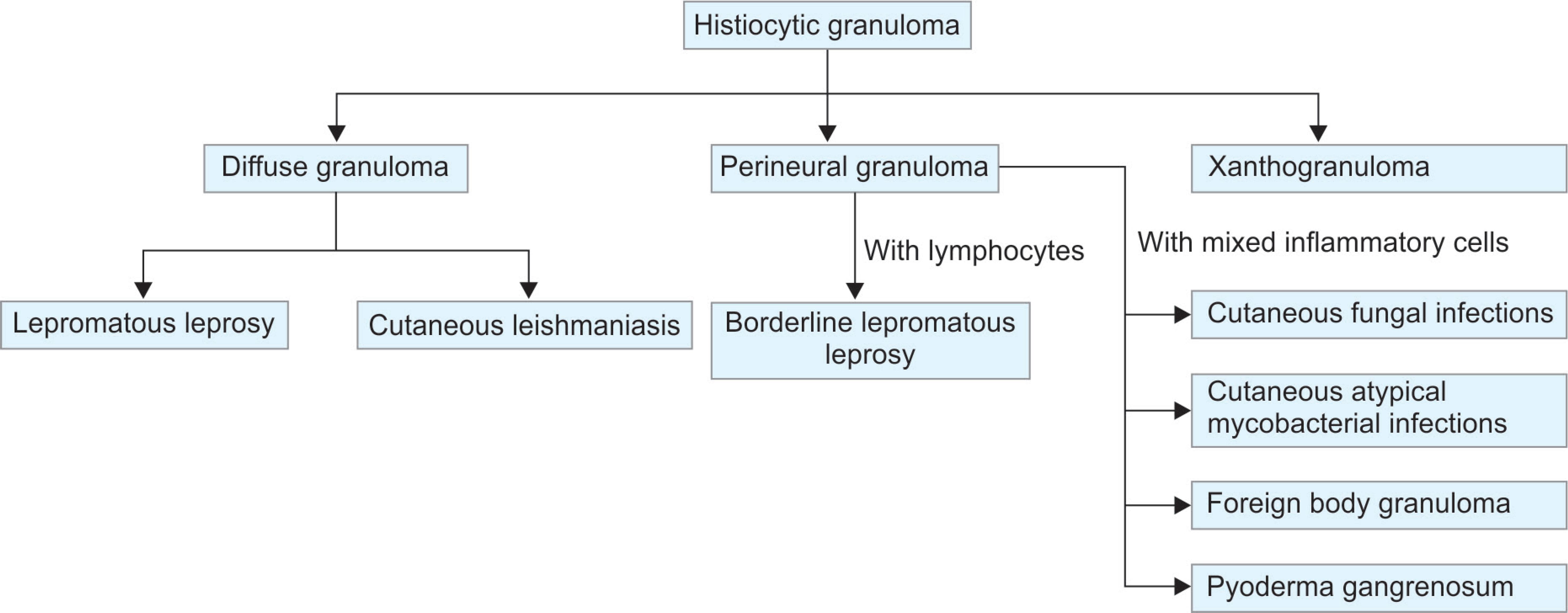
Flowchart 4: Algorithmic approach to diagnosis of histiocytic granulomas

Fig. 1: Microscopic picture of borderline tuberculoid leprosy with discrete epithelioid cell granuloma (arrow) in the dermis (HandE, 10×). Inset shows a perineural granuloma (HandE, 40×)
Lichen scrofulosorum is a rare tuberculid characterized by minute papular lesions with or without active tuberculosis. Histologically, the papillary dermis shows replacement of hair follicles by tuberculoid granulomas. Ziehl–Neelsen stain is usually negative for AFB. However, mycobacterial deoxyribonucleic acid (DNA) has been detected by PCR in few lesions.12
Syphilitic granulomas are usually found in the tertiary stage of the disease, although they are uncommon in lesions of the secondary phase. Lesions of secondary syphilis usually present as papulosquamous eruptions. Moreover, this rare infection may present with unusual clinical features and varied histopathological appearances, thus posing a diagnostic challenge. Nonspecific lymphoplasmacytic infiltrates are usually found in the superficial and deep layers with or without the presence of epithelioid granulomas. As the spirochetes are not consistently visualized on Warthin–Starry stain, serology is confirmatory for diagnosis.13
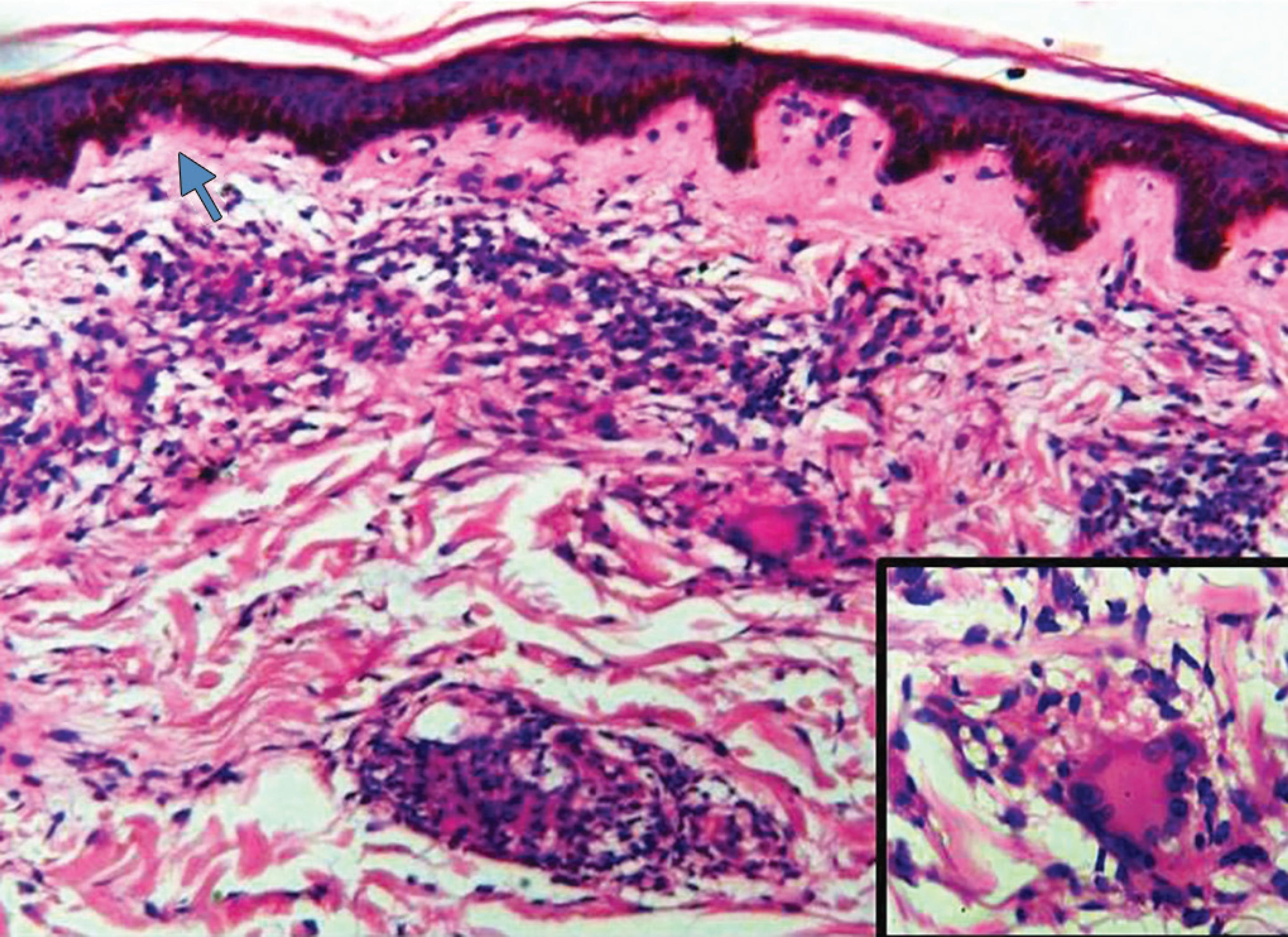
Fig. 2: Microscopic picture of polar tuberculoid leprosy with epithelioid cell granulomas and dense lymphocytic infiltration extending into the basal layer (arrow) (HandE, 10×). Inset shows an epithelioid cell granuloma (HandE, 40×)
Histoplasma capsulatum is a species of dimorphic fungi that is distributed worldwide and is found in soil contaminated by bird and bat droppings. The lesions present as nodules, abscesses, ulcers, and usually seen in immunocompromised individuals. Histological sections show a variable inflammatory cell infiltrate comprising foamy histiocytes and granulomas within the dermis. The histology often varies depending on the immunity of the host. The fungal organisms may be identified as multiple round spores with the surrounding clear halo intracellularly and extracellularly.14
Cryptococcus neoformans is found in contaminated pigeon excreta and soil. The skin lesions are usually part of a systemic infection. The patients are invariably immunocompromised. Histologically, the dermis shows dense inflammatory infiltrate comprising foamy histiocytes with or without a granuloma. The fungal organisms are identified as round to oval bodies with a clear halo seen within the macrophages and giant cells, highlighted by mucicarmine stain.15
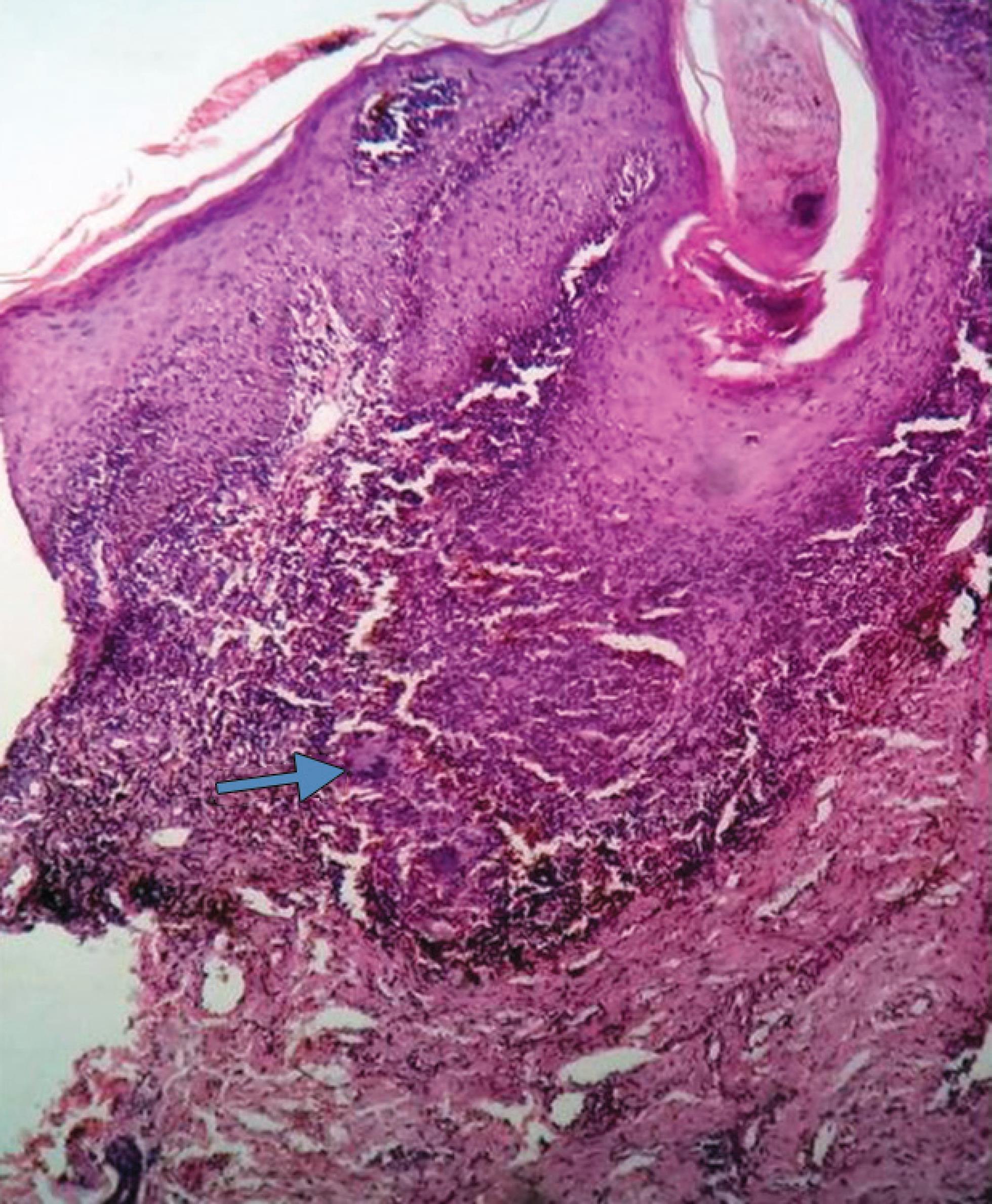
Fig. 3: Microscopic picture of lupus vulgaris with dermoepidermal junction showing granulomas (arrow) composed of epithelioid cells, histiocytes, lymphoplasmacytic infiltration, and giant cells (HandE, 10×)
Cutaneous leishmaniasis is transmitted by the bite of an infected phlebotomine sandfly and manifests within few weeks to months after travel to the regions of Central and South America, the Mediterranean basin, Africa, India, and the Middle East and Central Asia. The lesions are itchy small red papules with raised edges in skin-exposed areas which eventually ulcerate. The histological appearances vary, depending on the host response and the time of biopsy. Initial stages show collection of dermal macrophages with mild lymphoplasmacytic infiltrate followed by epidermal hyperplasia with ulceration in late stages. Over the subsequent months, there is a gradual decline in the number of macrophages and amastigotes, thus leaving behind dense dermal granulomatous inflammation comprising epithelioid cells, giant cells, and lymphocytes. Uniform round organisms are seen in the cytoplasm of the histiocytes. These are called Leishmania–Donovan bodies (amastigotes) and are usually stained with HandE and Giemsa.16
Granulomatous acne rosacea is a variant of acne rosacea, distinguished by eruption of erythematous and indurated yellowish brown papules over the face. The etiopathogenesis is unknown. There is a suggested role of type IV hypersensitivity reaction against Demodex folliculorum, keratinized cells and pilosebaceous units. Histologically, there are epithelioid granulomas with or without caseating necrosis, similar to lupus miliaris disseminatus faciei, which is a rare caseating granulomatous condition presenting as inflammatory or flesh-colored papules with a symmetrical distribution across the eyelids, nose, and upper lip.17
Cutaneous lesions in sarcoidosis assume a wide range of morphologies. Specific lesions are maculopapular eruptions, subcutaneous nodules, infiltrating plaques, scar infiltrations, and lupus pernio. Histologically, there are “naked” epithelioid granulomas in the dermis, which are devoid of lymphohistiocytic infiltrates with unusual necrosis. These granulomas may contain asteroid or Schaumann bodies. Asteroid bodies are eosinophilic, stellate, or spider-like inclusions, and Schaumann bodies are basophilic, laminated, rounded conchoidal calcified structures. Any infectious etiology can be ruled out by the use of ZN and periodic acid-Schiff (PAS) stains.18
Cutaneous lesions of metastatic Crohn’s disease present as nodules, papules, plaques, and ulcers. These lesions may involve the face, upper and lower limbs, the abdominal wall as well as the genitalia, inguinal, and perineal areas. Histologically, the dermis shows many sterile noncaseating granulomas composed of epithelioid histiocytes and Langhans giant cells accompanied by significant lymphoplasmacytic infiltration.19
In orofacial granulomatosis, individuals have a tenacious nontender labial swelling and numerous ulcers in the oral cavity, a variety of orofacial features. Histopathological examination of representative lesions shows noncaseating granulomas with submucosal edema. A greater fraction of these patients will be subsequently diagnosed as Crohn’s disease, with few of them being diagnosed as sarcoidosis. The remaining will be diagnosed as part of systemic infections or developing allergic reactions to food additives or drugs.20
PALISADED GRANULOMAS
Palisaded granulomas are found in various skin lesions such as granuloma annulare, necrobiosis lipoidica, rheumatoid nodule, gout, necrobiotic xanthogranuloma, palisaded and neutrophilic dermatosis, and papulonecrotic tuberculid.21
Granuloma annulare is a rare noninfectious, granulomatous skin disease characterized by erythematous papular lesions in an annular configuration. Histopathological examination reveals interstitial or palisaded pattern of histiocytic infiltrate around a central area of altered collagen. The infiltrate can extend up to mid-dermis (most common) or may involve only the upper dermis or entire dermis. The absence of well-formed granulomas cannot rule out granuloma annulare. Special stains such as Alcian blue are used to demonstrate mucin in the dermis.22
Necrobiosis lipoidica is a chronic granulomatous condition associated with diabetes mellitus. Patients present clinically with yellowish brown atrophic telangiectatic sclerodermiform plaques with elevated violaceous borders present on the shins. Histologically, there are interspersed interstitial, palisading granulomas with a “tiered” appearance accompanied by significant lymphocytic infiltrate involving the dermis. The absence of interstitial mucin deposits within the palisading granulomas of necrobiosis lipoidica differentiates it from granuloma annulare.23
In rheumatoid disease, patients frequently have subcutaneous rheumatoid nodules. Histological sections studied from these nodules show reticular dermis filled with extensive fibrinoid necrosis and fat surrounded by elongated histiocytic cells with focal palisaded arrangement.24
Gout is a disorder of uric acid metabolism. Painful dermal and subcutaneous nodules (tophi) usually in distal interphalangeal joint are the frequent clinical presentation. Histolopathological examination reveals pale granular material with needle-like clefts arranged radially, thus representing the dissolved urate crystals surrounded by palisading histiocytes. These crystals are preserved in specimens fixed in absolute alcohol, and they appear black when stained with von Kossa stain.25
Necrobiotic xanthogranuloma is an uncommon condition characterized by yellow-colored papules and nodules that amalgamate to form indurated and ulcerated xanthomatized plaques with a predilection for the periorbital area. Monoclonal paraproteins are detected in 75–80% of cases. Histologically, the dermis is replaced by a granulomatous infiltrate composed of foamy histiocytes, lymphocytes, and plasma cells along with many Touton and foreign body giant cells, surrounding foci of necrobiosis. These lesions are histologically similar to necrobiosis lipoidica.26
Palisaded neutrophilic and granulomatous dermatitis is seen in patients with a wide range of coexisting disorders such as autoimmune thyroid disease, rheumatoid arthritis, and lupus and inflammatory bowel disease. These symmetrical lesions are violaceous to erythematous papules or plaques often seen on the trunk. Initial histological features are characterized by a diffuse inflammatory infiltrate composed of polymorphs and nuclear debris in the dermis, with dermal collagen degeneration associated with leukocytoclastic vasculitis. Later histological features are similar to those of interstitial granuloma annulare.27
SUPPURATIVE GRANULOMAS
Suppurative granulomas are observed in atypical mycobacterial infections, fungal infections, pyoderma gangrenosum, and ruptured infected epidermoid cysts.28
“Swimming pool” granuloma caused by Mycobacterium marinum is a common cutaneous manifestation of atypical mycobacteria. Individuals usually present with single to multiple verrucous plaques or nodules on the hands and forearms that occur a fortnight following minimal skin trauma and subsequent exposure of the injured skin to the insulting aquatic conditions. Histologically, the epidermis shows features of pseudoepitheliomatous hyperplasia with a suppurative granulomatous inflammation in the dermis.29
The histopathological features of cutaneous fungal infections such as chromoblastomycosis, phaeohyphomycosis, sporotrichosis, and coccidioidomycosis are similar to epidermis showing features of pseudoepitheliomatous hyperplasia and ulceration; the dermis shows dense inflammation with suppurative granulomas. The fungal organisms are stained with Grocott or PAS stain. The species is determined by the morphology and culture.30
Superficial granulomatous pyoderma gangrenosum is an uncommon variant that is often mistaken for an infective dermatosis. It usually presents as a superficial ulcer on the trunk and may occur on surgical scars. Histologically, the epidermis shows features of hyperplasia with ulceration; acute inflammation with granulation tissue and ill-formed granulomas with surrounding necrotic debris. The histology often simulates a perforating granuloma annulare.31,32

Fig. 4: Microscopic picture showing a palisading epithelioid granuloma (arrow) with areas of stellate necrosis (star) (HandE, 10×)
Stellate necrosis with epithelioid granuloma (palisading or diffuse) admixed with neutrophils is often associated with actinomycosis, cat-scratch disease, lymphogranuloma venereum, Nocardia, Toxoplasma gondii, and other parasitic and fungal infections (Fig. 4).
XANTHOGRANULOMATOUS REACTIONS
Xanthogranulomatous reactions include juvenile xanthogranuloma (JXG), xanthomas, reticulohistiocytoma, leishmaniasis, cryptococcus, histoplasmosis, and lepromatous leprosy.
Classic JXG manifests as single to multiple yellowish-brown nodules. The dermis shows a cluster of foamy macrophages, foreign body type, and Touton giant cells with scattered lymphocytes (Fig. 5). These lesions are a part of non-Langerhans cell histiocytoses, which are clinically diverse and categorized according to the clinical presentation, histology, immunohistochemistry, and ultrastructural profiles. The lesions stain positive for CD68, show variable staining patterns for factor XIIIa, and are negative for S100 protein and CD1a.33,34
Xanthomas are associated with abnormalities of lipoprotein metabolism and appear variably as eruptive, planar, tendinous, tuberous, or verruciform lesions. Xanthelasma are planar xanthomas appearing as yellowish brown plaques over the eyelids. Histological examination shows the dermis with collection of pale foamy histiocytes.35
Reticulohistiocytoma usually presents as a single yellowish-red colored nodule on the head and neck of adults. Histological sections show large histiocytes admixed with Touton giant cells and interspersed lymphocytes infiltrating the dermis. These cells have PAS-positive ground glass cytoplasm. Similar findings are observed in rare multicentric reticulohistiocytosis associated with severe arthropathy.36
Lepromatous leprosy is seen in individuals who do not have a cell-mediated immune response to the lepra bacilli. In borderline lepromatous leprosy, multiple histiocytic granulomas are seen predominantly around the nerves. In polar lepromatous leprosy, the dermis is filled with sheets of foamy macrophages owing to accumulation of mycobacteria, which surround the adnexal glands and nerve bundles and do not tend to form granulomas (Fig. 6). Lepra bacilli are abundant with Fite-Faraco stain (Fig. 7).37
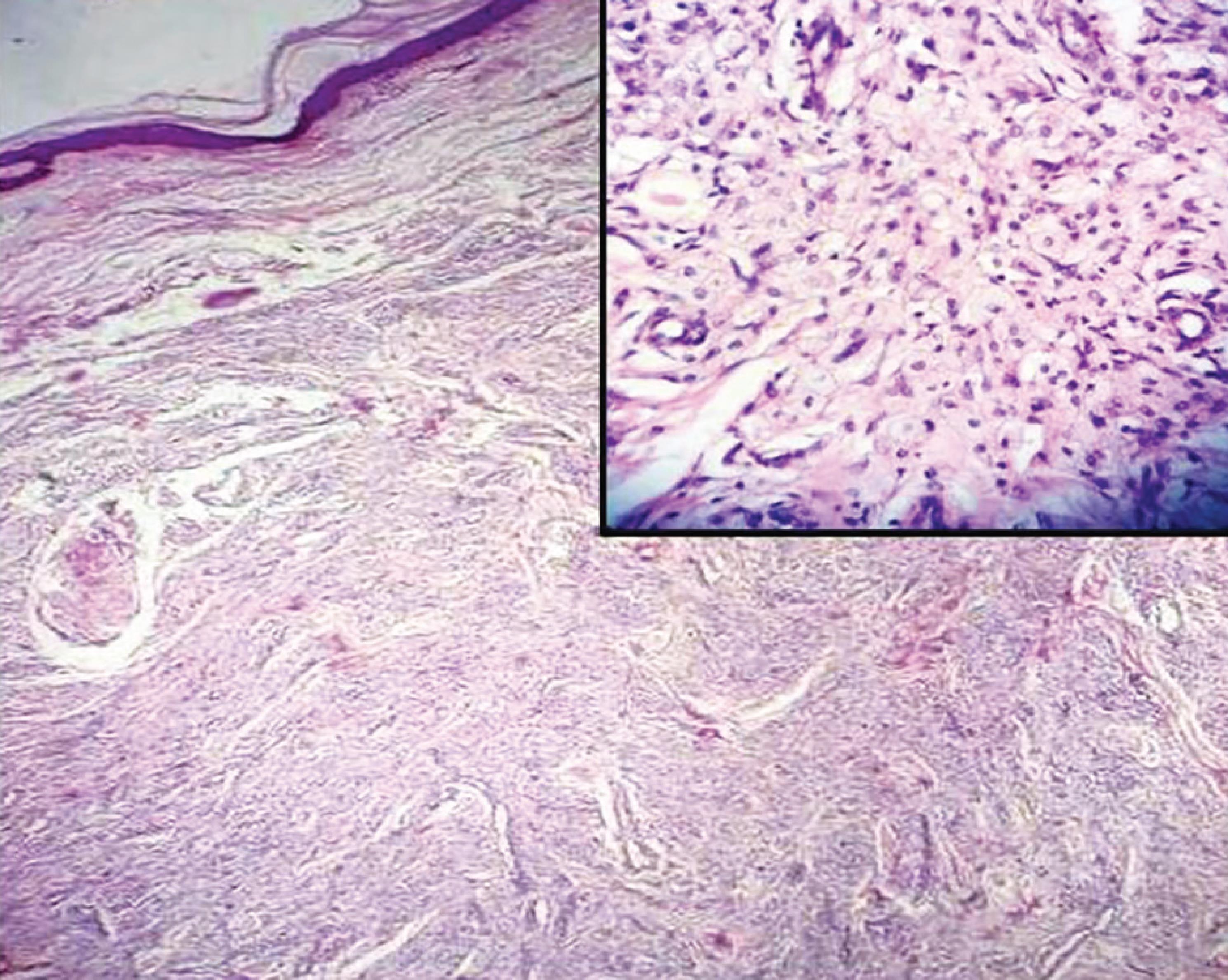
Fig. 5: Microscopic picture of juvenile xanthogranuloma with dermal infiltrate (HandE, 4×). Inset shows mass composed of sheets of foamy macrophages admixed with inflammatory cells infiltrates (HandE, 40×)

Fig. 6: Microscopic picture of lepromatous leprosy with clear subepidermal Grenz zone and underlying collection of foamy macrophages (HandE, 10×). Inset shows foamy macrophages (HandE, 40×)
| Cosmetic fillers |
| Collagen |
| Calcium hydroxylapatite |
| Hyaluronic acid |
| Paraffin |
| Silicone |
| Drugs and medications |
| Anabolic corticosteroids |
| Hepatitis B vaccine |
| Hydroquinone |
| Leuprorelin acetate |
| Pentazocine |
| Polyvinylpyrrolidone |
| Zinc-containing insulin |
| Mineral, metallic, and other particles |
| Aluminum |
| Beryllium |
| Mercury |
| Nickel |
| Titanium |
| Tattoo pigments |
| Miscellaneous |
| Arthropod fragments |
| Cactus bristles and other plants |
| Glass |
| Splinters |
| Synthetic fibers |
FOREIGN BODY GRANULOMAS
Granulomatous foreign body reactions are variable. The lesion usually presents as a erythematous nodule at the site of prior inoculation of exogenic material or deposition of endogenous material. Various foreign substances found in skin are enlisted in Table 1.

Fig. 7: Microscopic picture of lepromatous leprosy showing positive Fite-Faraco stain for lepra bacilli with bacterial index 6+. Globi are shown (arrow) (Fite-Faraco stain 100×)

Fig. 8: Microscopic picture of foreign body granuloma composed of foreign body giant cells (arrow) with necrosis and mixed inflammatory cell infiltrates (HandE, 10×). Inset shows a foreign body giant cell with material (arrow) (HandE, 40×)
A ruptured epidermoid cyst could also give a similar histopathological picture. Histological sections show a granulomatous inflammation composed of histiocytes with foreign body giant cells, distinguished by cytoplasm showing irregular scattering of nuclei (Fig. 8). This histomorphological picture is often mistaken for palisading, suppurative and sarcoid granulomas. In few instances, the foreign bodies that are not picked up on HandE stain should alternatively be visualized under polarized light or detected with the help of special stains such as Masson trichrome for animal collagen.38
MISCELLANEOUS GRANULOMATOUS CONDITIONS
Lichen nitidus presents as tiny shiny papules over the forearms, trunk, and genitalia, histological sections show atrophic and parakeratotic epidermis claw-like pattern of curvilinear, finger-like extensions with the papillary dermis showing lymphocytic and histiocytic infiltrates admixed with multinucleated giant cells accompanied by basal vacuolar degeneration.39
Actinic granulomas are usually incidental findings in skin excision biopsies from the dorsum of the hands, head, and neck. There is significant damage to the skin owing to sun exposure. Histological sections show remarkable solar elastosis with broken elastic fibers in the center of ill-formed granulomas with multinucleated giant cells in the upper dermis.40
Approximately 50% of the patients having Churg–Strauss granulomatosis have cutaneous presentation of subcutaneous nodules on scalp and extremities that have the tendency to ulcerate. Histological features of necrotizing granulomatous vasculitis with large numbers of eosinophils are present. Extravascular necrotizing granulomas may also be found.41,42
Approximately 50% of the patients with Wegener’s granulomatosis have painful subcutaneous nodules and punched out ulcers. Histological sections show necrotizing granulomatous vasculitis with blood vessels at various phases of involvement. At least 75–80% of the patients test positive for antineutrophil cytoplasmic antibodies.43,44
Neural granulomas are hallmark of both tuberculoid and lepromatous variants of leprosy.45
Erythema nodosum is associated with rheumatoid arthritis, inflammatory bowel disease, infection, sarcoidosis, drugs, and pregnancy. It manifests as painful nodular lesions on the ankles, knees, and shins with no ulceration, and the lesions heal without formation of a scar. Histological sections show features of septal panniculitis with broad septa containing inflammatory infiltrate depending upon the age of the lesion. Initial stages show edema, hemorrhage, and neutrophils, which are superseded by features of fibrosis, periseptal granulation tissue, lymphocytes, and multinucleated giant cells in late stages of the disease. In addition to this, there are septal collections of tiny histiocytes as nodular aggregates surrounding a cleft called “Miescher’s radial granulomas.”46
Erythema induratum or nodular vasculitis which may be associated with tuberculosis presents as panniculitis with tender, erythematous nodules and ulcerative plaques present on the lower limbs that can heal with scar formation. Histological features of vaguely granulomatous inflammation with vasculitis and lobular panniculitis are observed.47
Immunodeficiency-associated granulomas are found in immunocompromised individuals who are highly susceptible to infections. The presence of noncaseating granulomas in the absence of infection is observed in individuals with primary immunodeficiency disorders and common variable immunodeficiency.48
MALIGNANCIES SIMULATING BENIGN GRANULOMATOUS REACTIONS
Granulomatous components are often encountered in malignant diseases. In patients with an underlying malignancy, the ontogenesis of noninfectious granulomas at loci with no evidence of metastasis is a well-known phenomenon but skin involvement is often rare.49,50
Epithelioid sarcoma is an unusual high-grade soft tissue malignancy with a predilection for adolescent males. Superficially located tumors usually presents as solitary or multiple firm, slow growing, painless nodules over the extremities. Deep-seated lesions could occur associated with significant skin ulceration. The histopathological sections show tumor cells with epithelioid appearance arranged in a granuloma-like fashion mixed with spindle cells around areas of necrosis and central hyalinization. Individual tumor cells have vesicular and pleomorphic nuclei with occasionally prominent nucleoli. The tumor’s histology is often confused with that of a deep granuloma annulare.51
Langerhans cell histiocytosis (LCH; histiocytosis X) is an infrequently occurring idiopathic condition involving a clonal proliferation of Langerhans cells. Histopathological examination reveals epidermis and upper dermis showing a dense infiltration of eosinophils mixed with large, polygonal Langerhans cells with abundant, granular, indistinct, and mildly eosinophilic cytoplasm with grooved nuclei with indented nuclear membranes. The neoplastic cells stain positive for S100 protein and CD1a. Electron microscopic examination reveals pentalaminar intracytoplasmic structures called Birbeck granules. Langerhans cell histiocytosis usually affects children with a predilection for males. It has diverse clinical presentation, of which, cutaneous manifestations are common.52
Mycosis fungoides is a peripheral T cell lymphoma derived from mature, post-thymic T lymphocytes, presenting as cutaneous patches that eventually progressed to plaques, tumors, and erythroderma. It affects predominantly older adults with higher incidence in men than women. Early lesions are erythematous patches on the buttock, trunk, and proximal limbs of males and breasts in females. These patches are heterogenous in size and shape with fine scales and variable atrophy. A definitive histopathological diagnosis is not possible in the initial stages. The plaque stage is characterized by acanthotic epidermis with band-like papillary dermal lymphoid infiltrate possessing cerebriform nuclei and features of epidermotropism. A quarter of these cases show “Pautrier’s micro-abscesses.” An uncommon histologic subtype is the granulomatous variant characterized by non-necrotizing epithelioid granulomas admixed with a lymphomatous epidermal and dermal infiltrate, simulating a granuloma annulare.53,54
Lymphomatoid granulomatosis is an uncommon angiocentric and angiodestructive lymphoproliferative disease involving extranodal sites, composed of B cells positive for Epstein–Barr virus, which are admixed with reactive T cells. It is a disease of adult males. The skin lesions can be in the form of ulcerative red papules, indurated plaques, and subcutaneous nodules. Histological sections show angiocentric and angiodestructive polymorphous lymphoid infiltrate composed of lymphocytes admixed with plasma cells, immunoblasts, and histiocytes rarely accompanied by neutrophils and eosinophils, associated with or without ill-formed granulomas and focal areas of necrosis.55,56
DIAGNOSTIC ROLE OF SEROLOGICAL TESTS AND ANCILLARY TECHNIQUES
Serological tests such as enzyme-linked immunosorbent assay and ancillary techniques such as PCR are useful in defining the diagnosis of granulomatous skin diseases such as cutaneous tuberculosis and borderline leprosy in suspected patients with clinically suggestive lesions presenting with inconclusive histopathology and negative bacilli on sections, which may be owing to low levels of bacillary load.57,58 In these methods, a disease causing suspect pathogen is identified by detection of antigens and antibodies or by targeting the mere presence of nucleic acids. These tests are also helpful in the diagnostic workup of autoimmune granulomas. Few serological tests and ancillary techniques that can be used for diagnosis of infectious and noninfectious granulomatous skin diseases are summarized in Table 2.59–66
| Granulomatous skin disease | Serological tests | Ancillary techniques |
|---|---|---|
| Leprosy | ELISA using Mycobacterium leprae cell wall PGL-I and SACT based on the 35 kDa protein antigens of M. leprae | PCR |
| Cutaneous tuberculosis | QuantiFERON and ELISpot measure the amount of interferon-gamma released by lymphocytes confronted with Mycobacteriumtuberculosis-specific antigens | PCR |
| Cutaneous leishmaniasis | IFA, ELISA, LFA, and DAT | Western blot and PCR used for detection and defining species |
| Syphilis | A combination of treponemal tests (FTA-Abs and TPPA) and nontreponemal tests (VDRL and RPR tests) | PCR in 39–69% of biopsies |
| Cutaneous histoplasmosis | IDT, CFT, EIA in urine, and serum | PCR |
| Cutaneous cryptococcosis | CrAg, LA, and EIA | PCR |
| Rheumatoid nodule | RF, anti-CCP, and anti-MCV | Cytology, histopathology, IHC, and molecular diagnostics |
| DLE | ELISA for detection of ANA, anti-dsDNA, and anti-RNP | Histopathology, IF, and molecular diagnostics |
ELISA, enzyme-linked immunosorbent assay; PGL-I, phenolic glycolipid-I; SACT, serum antibody competition test; kDa, kilo Dalton; PCR, polymerase chain reaction; ELISpot, enzyme-linked immuno-absorbent spot; IFA, indirect fluorescent antibody; LFA, lateral flow assay; DAT, direct agglutination test; FTA-Abs, fluorescent treponemal antibody absorption; TPPA, Treponema pallidum particle agglutination assay; VDRL, venereal disease research laboratory; RPR, rapid plasma reagin; IDT, immunodiffusion test, CFT, complement fixation test; EIA, enzyme immunoassay; CrAg, cryptococcal polysaccharide capsular antigen; LA, latex agglutination; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptides; anti-MCV, anti-mutated citrullinated vimentin; IHC, immunohistochemistry; DLE, discoid lupus erythematosus; ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA; anti-RNP, anti-ribonucleoprotein; IF, immunofluorescence
CONCLUSION
Granulomatous reaction patterns are frequently encountered in dermatopathology and results from several etiologies. An apt clinical history, meticulous histolopathological evaluation, and good clinicopathological correlation are of utmost importance in arriving at the final diagnosis. Culture of microbes, special stains, and molecular methods for detection of nuclear antigens and nucleic acids may also be necessary. However, the diagnosis could be inconclusive in a minimal amount of cases. With the passage of time, it is expected that more etiological agents will be recognized, with the ontogeny of unknown adverse drug reactions and reactions to various treatment modalities. The incidence of uncommon infections in immunocompromised individuals may increase. The advancements in molecular methods may give us a helping hand in expanding the spectrum and improving the diagnostics and therapeutics in granulomatous dermatoses.
REFERENCES
1. Gupta K, Kumari A, Mangal K. Granulomatous lesions: a diagnostic challenge to dermatopathologists. Int J Med Res Prof 2016;2(4):33–39.
2. Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Mycobact Dis 2017;7:1–12. DOI: 10.1016/j.jctube.2017.02.001.
3. Terziroli Beretta-Piccoli B, Mainetti C, Peeters M-A, Laffitte E. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol 2018;54(1):131–146. DOI: 10.1007/s12016-017-8666-8.
4. Mysore V. Fundamentals of pathology of skin, B.I. Publications; 2008. pp. 96–99.
5. Blessing K. Cutaneous granulomatous inflammation. Curr Diagn Pathol 2005;11(4):219–235. DOI: 10.1016/j.cdip.2005.05.001.
6. Yadav D, Ramam M. Epithelioid cell granuloma. Indian J Dermatopathol Diagn Dermatol 2018;5(1):7. DOI: 10.4103/ijdpdd.ijdpdd_13_18.
7. Lastória JC, Abreu MA. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects - part 1. An Bras Dermatol 2014;89(2):205–218. DOI: 10.1590/abd1806-4841.20142450.
8. Mysore V. Fundamentals of pathology of skin, B.I. Publications; 2008. p. 86.
9. Roy P, Dhar R, Patro P, et al. Histopathological study of leprosy patients in a tertiary care hospital in Navi Mumbai. Int J Health Sci Res 2019;9(2):6–12.
10. Nadia S, Rashmi J, Sohaib A, Rawat S, Thamarai NS, Meena H. Clinico pathological correlation of leprosy: a 4 years retrospective study from a tertiary referral centre in north India. Int J Med Res Health Sci 2015;4(2):350. DOI: 10.5958/2319-5886.2015.00065.X.
11. Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthetic Dermatol 2009;2(10):19–27.
12. Molpariya A, Ramesh V. Lichen scrofulosorum: importance of early recognition. Clin Exp Dermatol 2017;42(4):369–373. DOI: 10.1111/ced.13050.
13. Lee GL, Gru AA, Wong HK, Nagarajan P. Granulomatous syphilis: a pattern to remember. Case report and review of literature. Clin Microbial 2015;4:184.
14. Raina R, Mahajan V, Sood A, Saurabh S. Primary cutaneous histoplasmosis in an immunocompetent host from a nonendemic area. Indian J Dermatol 2016;61(4):467. DOI: 10.4103/0019-5154.185748.
15. Leão CA, Ferreira-Paim K, Andrade-Silva L, Mora DJ, da Silva PR, Machado AS, et al. Primary cutaneous cryptococcosis caused by Cryptococcus gattii in an immunocompetent host. Med Mycol 2011;49(4):352–355. DOI: 10.3109/13693786.2010.530697.
16. Hepburn N. Cutaneous leishmaniasis: an overview. J Postgrad Med 2003;49(1):50. DOI: 10.4103/0022-3859.928.
17. Kaur S, Kanwar AJ, Thami GP, Mohan H, Arya SK. Granulomatous rosacea: is it a variant of lupus miliaris disseminatus faciei? Indian J Dermatol Venereol Leprol 2003;69 (Suppl S1):58–60.
18. Bharathi M, Ravikumar T. Isolated cutaneous sarcoidosis: a new insight into the old entity. J Clin Diagn Res 2013;7(8):1725.
19. Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012;136(3):329–332. DOI: 10.5858/arpa.2010-0666-RS.
20. Rana A. Orofacial granulomatosis: a case report with review of literature. J Indian Soc Periodontol 2012;16(3):469. DOI: 10.4103/0972-124X.100934.
21. Rosińska-Więckowicz A, Bowszyc-Dmochowska M. Granulomatous skin disease with a histological pattern of palisading granuloma - an atypical facial necrobiosis lipoidica or more? Postepy Dermatol Alergol 2017;34(6):618–621. DOI: 10.5114/ada.2017.72468.
22. Chatterjee D, Kaur M, Punia RPS, Bhalla M, Handa U. Evaluating the unusual histological aspects of granuloma annulare: a study of 30 cases. Indian Dermatol Online J 2018;9(6):409–413.
23. Thomas M, Khopkar US. Necrobiosis lipoidica: a clinicopathological study in the Índian scenario. Indian Dermatol Online J 2013;4(4):288–291. DOI: 10.4103/2229-5178.120639.
24. García-Patos V. Rheumatoid nodule. Semin Cutan Med Surg 2007;26(2):100–107. DOI: 10.1016/j.sder.2007.02.007.
25. Orzan OA, Simion G, Tudose I, Costache M, Giurcăneanu C. Histopathological study of cutaneous lesions in gout. Rom J Diabetes Nutr Metab Dis 2013;20(1):11–19.
26. Pokharel A, Koirala I. Necrobiotic granuloma: an update. Indian J Dermatopathol Diagn Dermatol 2018;5(1):27. DOI: 10.4103/ijdpdd.ijdpdd_12_18.
27. Kalen JE, Shokeen D, Ramos-Caro F, Motaparthi K. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep 2017;3(5):425–428. DOI: 10.1016/j.jdcr.2017.06.010.
28. Thomas M, Rao R, Kumar GN. An overview of suppurative granuloma. Indian J Dermatopathol Diagn Dermatol 2018;5(1):19. DOI: 10.4103/ijdpdd.ijdpdd_19_18.
29. Sette CS, Wachholz PA, Masuda PY, da Costa Figueira RBF, de Oliveira Mattar FR, Ura DG. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Trop Dis 2015;21:7. DOI: 10.1186/s40409-015-0008-9.
30. Fernandez-Flores A, Saeb-Lima M, Arenas-Guzman R. Morphological findings of deep cutaneous fungal infections. Am J Dermatopathol 2014;36(7):531–556. DOI: 10.1097/DAD.0b013e31829cc6f3.
31. Persing SM, Laub D. Superficial granulomatous pyoderma of the face: a case report and review of the literature. Eplasty 2012;12:e56.
32. Kumar S, Vinay K, Parsad D, Saikia U, Kumaran M. Superficial granulomatous pyoderma: a great mimicker. Indian J Dermatol Venereol Leprol 2018;84(3):374. DOI: 10.4103/0378-6323.196317.
33. Cypel TKS, Zuker RM. Juvenile xanthogranuloma: Case report and review of the literature. Can J Plast Surg J Can Chir Plast 2008;16(3):175–177. DOI: 10.1177/229255030801600309.
34. Szczerkowska-Dobosz A, Kozicka D, Purzycka-Bohdan D, Biernat W, Stawczyk M, Nowicki R. Juvenile xanthogranuloma: a rare benign histiocytic disorder. Postepy Dermatol Alergol 2014;31(3):197–200. DOI: 10.5114/pdia.2014.40918.
35. Pai V, Shukla P, Bhobe M. Combined planar and eruptive xanthoma in a patient with type lla hyperlipoproteinemia. Indian J Dermatol Venereol Leprol 2014;80(5):467. DOI: 10.4103/0378-6323.140323.
36. Bansal M, Manchanda K, Pandey S. Multiple cutaneous reticulohistiocytoma in middle aged female. Indian Dermatol Online J 2014;5(1):74. DOI: 10.4103/2229-5178.126040.
37. Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol 2015;33(1):38–45. DOI: 10.1016/j.clindermatol.2014.10.003.
38. Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin 2015;33(3):497–523. DOI: 10.1016/j.det.2015.03.014.
39. Mehta V, Balachandran C. Generalized lichen nitidus in childhood. Indian J Dermatol 2008;53(4):221. DOI: 10.4103/0019-5154.44794.
40. Yaghoobi R, Ranjbari N, Feily A. Actinic granuloma. Dermatol Pract Concept 2014;4(3):4. DOI: 10.5826/dpc.0403a04.
41. Marques CC, Fernandes EL, Miquelin GM, Colferai MMT. Cutaneous manifestations of Churg-Strauss syndrome: key to diagnosis. An Bras Dermatol 2017;92 (5 Suppl 1):56–58. DOI: 10.1590/abd1806-4841.20175522.
42. Aberer E, Dekan G, Mayr-Kanhäuser S, Aberer W. Extravascular necrotizing granuloma induced by mineral wool in circumscribed scleroderma lesions. Exog Dermatol 2002;1(5):253–259. DOI: 10.1159/000068800.
43. Comfere NI, Macaron NC, Gibson LE. Cutaneous manifestations of Wegener's granulomatosis: a clinicopathologic study of 17 patients and correlation to antineutrophil cytoplasmic antibody status. J Cutan Pathol 2007;34(10):739–747. DOI: 10.1111/j.1600-0560.2006.00699.x.
44. Nasir N, Ali SA, Mehmood Riaz HM. Cutaneous ulcers as initial presentation of localized granulomatosis with polyangiitis: a case report and review of the literature. Case Rep Rheumatol 2015;2015:1–7. DOI: 10.1155/2015/517025.
45. Shenoy N, Nair NG. Study of S100 immunostaining in demonstrating neural granulomas in paucibacillary leprosy. Indian J Dermatol 2018;63(3):215.
46. Requena L, Requena C. Erythema nodosum. Dermatol Online J 2002;8(1):4.
47. Gupta P, Saikia U, Arora S, De D, Radotra B. Panniculitis: a dermatopathologist’s perspective and approach to diagnosis. Indian J Dermatopathol Diagn Dermatol 2016;3(2):29. DOI: 10.4103/2349-6029.195224.
48. Abdelhakim S, Cafone J, Basak R. Cutaneous manifestations of primary immunodeficiency. Indian J Paediatr Dermatol 2017;18(3):155. DOI: 10.4103/ijpd.IJPD_10_17.
49. Chhabra S, Mohan H, Bal A. Granulomas in association with neoplasm: a reaction or a different primary process? J Postgrad Med 2009;55(3):234. DOI: 10.4103/0022-3859.57398.
50. Moreira C, Rios E, Baudrier T, Azevedo F. Cutaneous granulomatous reaction as the first manifestation of Hodgkin’s lymphoma. Rev Bras Hematol E Hemoter 2017;39(1):70–72. DOI: 10.1016/j.bjhh.2016.11.004.
51. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthetic Dermatol 2009;2(5):49–54.
52. Aruna DR, Pushpalatha G, Galgali S, Prashanthy. Langerhans cell histiocytosis. J Indian Soc Periodontol 2011;15(3):276–279. DOI: 10.4103/0972-124X.85675.
53. Akinbami AA, Osikomaiya BI, John-Olabode SO, Adediran AA, Osinaike O, Uche EI, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep 2014;7:95–98. DOI: 10.4137/CCRep.S15724.
54. Gallardo F, García‐Muret MP, Servitje O, Estrach T, Bielsa I, Salar A, et al. Cutaneous lymphomas showing prominent granulomatous component: clinicopathological features in a series of 16 cases. J Eur Acad Dermatol Venereol 2009;23(6):639–647. DOI: 10.1111/j.1468-3083.2008.03020.x.
55. Roschewski M, Wilson WH. Lymphomatoid granulomatosis. Cancer J 2012;18(5):469–474. DOI: 10.1097/PPO.0b013e31826c5e19.
56. Song JY, Pittaluga S, Dunleavy K, Grant N, White T, Jiang L, et al. Lymphomatoid granulomatosis--a single institute experience: pathologic findings and clinical correlations. Am J Surg Pathol 2015;39(2):141–156. DOI: 10.1097/PAS.0000000000000328.
57. Sengupta U. Serodiagnostic tests for leprosy. Indian J Clin Biochem 1997;12 (S1):93–96. DOI: 10.1007/BF02873072.
58. Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract 2018;2018:1–9. DOI: 10.1155/2018/7201973.
59. Guarner J. Detection of microorganisms in granulomas that have been formalin-fixed: review of the literature regarding use of molecular methods. Scientifica 2012;2012:1–16. DOI: 10.6064/2012/494571.
60. Mitteldorf C, Tronnier M. Histologic features of granulomatous skin diseases: infectious granulomatous disorders. J Dtsch Dermatol Ges 2016;14(4):378–387. DOI: 10.1111/ddg.12955.
61. de Vries HJC, Reedijk SH, Schallig HDFH. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol 2015;16(2):99–109. DOI: 10.1007/s40257-015-0114-z.
62. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol 2015;22(2):137–147. DOI: 10.1128/CVI.00681-14.
63. Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 2017;55(6):1612–1620. DOI: 10.1128/JCM.02430-16.
64. Egerer K, Feist E, Burmester G-R. The serological diagnosis of rheumatoid arthritis. Dtsch Arztebl Int 2009;106(10):159–163. DOI: 10.3238/arztebl.2009.0159.
65. Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol 2000;53(6):424–432. DOI: 10.1136/jcp.53.6.424.
66. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am 2016;30(1):179–206. DOI: 10.1016/j.idc.2015.10.006.
________________________
© The Author(s). 2019 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.